K2Cu3(Fe(CN)6)2 In Situ-Modified MXene Nanosheets for Selective Enrichment of Cs+ and the Mechanism
Jinchang Zhang1, Xuefeng Cheng1, Jicai Jiang1, Apiladda Pattanateeradetch2, Shuai Shi1, Chanat Chokejaroenrat2, Qingfeng Xu1(徐庆锋)*, Jianmei Lu1(路建美)*
1College of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, China
2Department of Environmental Technology and Management, Faculty of Environment, Kasetsart University, Bangkok 10900, Thailand
Inorg. Chem.2025, 64, 4920–4933
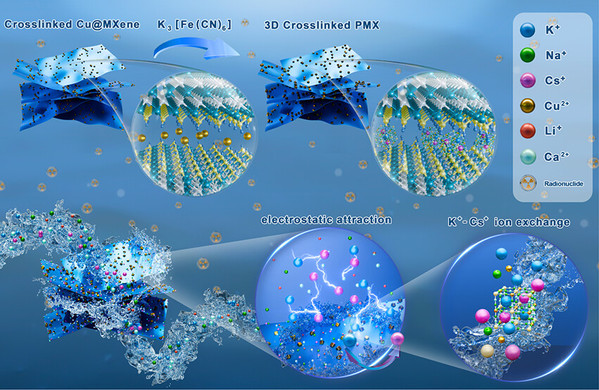
Abstract: Selective enrichment of cesium ions (Cs+) at ultralow concentrations is essential for resource recovery and radioactive waste disposal, yet efficient adsorbents are lacking. Herein, we reported a Prussian blue analogue (K2Cu3(Fe(CN)6)2, Cu-PBA) decorated on MXene nanosheets by in situ fabrication, forming a composite material termed PMX, for enhanced adsorption of Cs+ in acidic solutions and seawater. The stable, negatively charged MXene effectively anchors Cu2+ precursors and promotes Cs+ adsorption. The synergistic interaction between MXene and the in situ-synthesized Cu-PBA significantly enhances the adsorption performance and water stability of PMX in both acidic solutions and seawater. PMX achieves rapid adsorption equilibrium within 5 min, with a high adsorption capacity of 408.2 mg/g at pH 1, surpassing conventional adsorbents. Moreover, PMX shows excellent Cs+ selectivity (Kd= 68,361.7 mL/g), cycle stability, and notable anti-irradiation ability, demonstrating superior efficiency in Cs+ enrichment from complex matrices. The adsorption mechanism involves electrostatic attraction and K+/Cs+ ion exchange, facilitated by MXene’s functional groups and the Cu-PBA structure. These findings underscore the excellent potential of PMX as an efficient adsorbent for resource enrichment and the removal of radioactive elements such as Cs+.
Article information: //doi.org/10.1021/acs.inorgchem.4c04808