FeCl2·4H2O-Mediated Conversion of the CF3 Group into a Series of Esters: A Strategy for the Synthesis of FeII Complexes with In Situ-Formed Ester-Containing Ligands
Qian Zhang1, Xiaohan Yang1, Biqin Wang1, Yeye She1, Aron Szekely2,Yafei Li1, Yahong Li1(李亚红)*
1College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, People’s Republic of China
2Department of Chemistry, University of Waterloo, 200 University Ave W, Waterloo, ON N2L 3G1, Canada
Inorg. Chem.2025, 64, 4934–4946
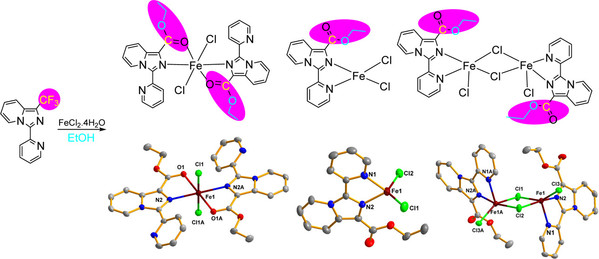
Abstract: A practical strategy for the preparation of a series of iron(II) complexes has been developed. This methodology features in situ esterification of the CF3 group on the backbone of the PIP–CF3 ligand (HPIP = 3-(pyridin-2-yl)imidazo[1,5-a]pyridine, PIP–CF3 = 3-(pyridin-2-yl)-1-(trifluoromethyl)imidazo[1,5-a]pyridine) by a wide range of alcohols. Treatment of FeCl2·4H2O with the PIP–CF3 ligand in EtOH under solvothermal conditions leads to the formation of complexes [Fe(PIP–COOEt)2Cl2] (1), [Fe2(PIP–COOEt)2Cl4] (2), and [Fe(PIP–COOEt)Cl2] (2′) (PIP–COOEt = ethyl 3-(pyridin-2-yl)imidazo[1,5-a]pyridine-1-carboxylate). EtOH serves as a solvent and is also involved in the esterification of the CF3 group. The esterification protocol features a broad substrate scope. The CF3 moiety of the PIP–CF3 ligand could be esterified by a wide range of alcohol substrates. Compounds [Fe(PIP–COOnPr)2Cl2] (3), [Fe2(PIP–COOnPr)2Cl4] (4), [Fe2(PIP–COOiPr)2Cl4] (5), and [Fe(PIP–CF3)2Cl2]·iPrOH (6·iPrOH) were isolated, and their structures were characterized. The mechanism for the esterification of the CF3 group was proposed by examining the conditions for the esterification transformations.
Article information: //doi.org/10.1021/acs.inorgchem.4c04876